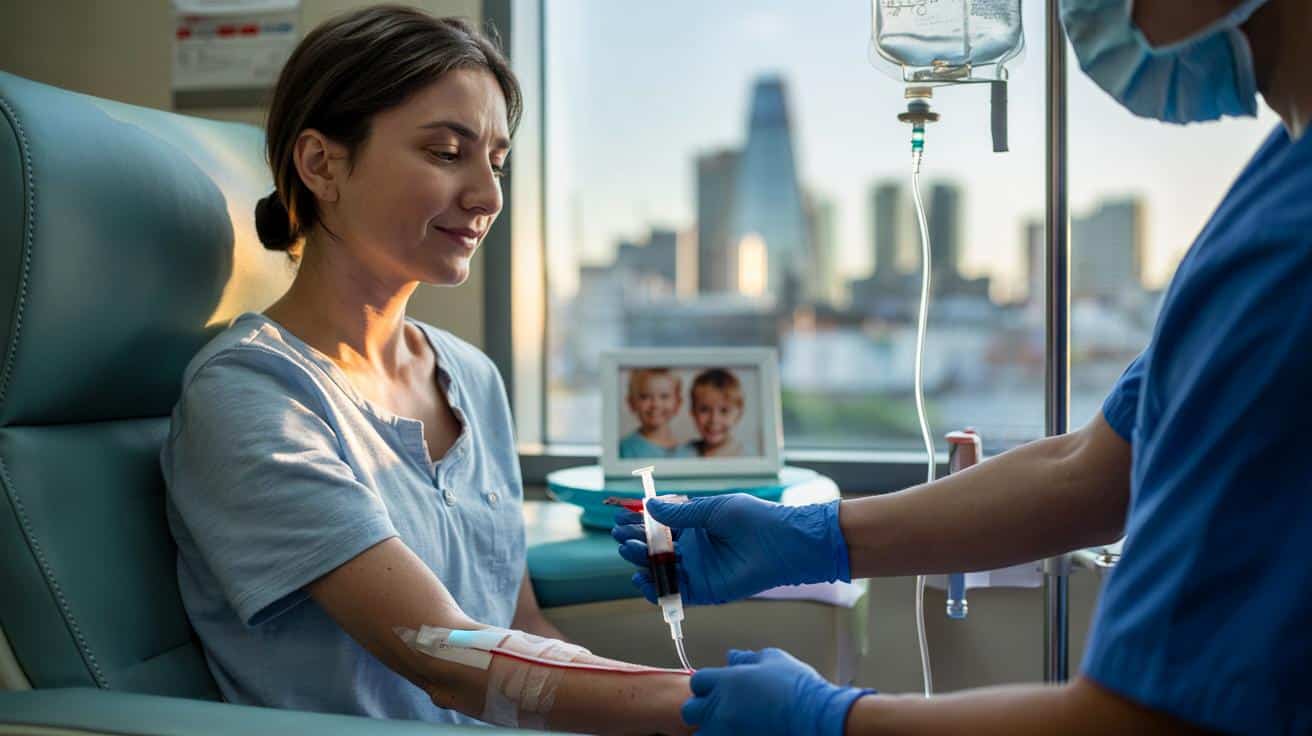
A Hertfordshire biology teacher, aged 37, has received a bespoke CAR T-cell infusion at University College London Hospital. Doctors gave the one-off treatment during a three‑minute procedure. The global trial, which plans to enrol up to 18 people by early 2027, will test whether a single dose can quieten multiple sclerosis for the long term.
A teacher’s path to the trial
Emily Henders learned she had multiple sclerosis during the 2021 festive period. Tingling in her hands came first. Subtle changes followed at work and at home. A misstep on a pavement. A tremor during a classroom experiment. She noticed, even when others did not.
Relapses accumulated. Standard therapy slowed things but did not stop them. She feared another episode would arrive without warning. She also feared a wheelchair in later years. She worried most for her two young sons, now six and four, who had already seen paramedics carry their mother away during a severe relapse.
Last week she sat in a chair at UCLH and received tailored immune cells from her own blood. She left the unit feeling steady. She said her energy returned, with no fever and no nausea in the days after the dose. Weekly checks will now track her recovery and the behaviour of the altered cells.
The infusion lasted roughly three minutes. The aim is long remission with a single treatment, not lifelong drugs.
What the one‑off therapy tries to do
Multiple sclerosis attacks the brain and spinal cord. The immune system mistakes healthy tissue and causes damage. Many current medicines dampen this response. CAR T-cell therapy offers a different route. It rewires a patient’s own T cells to recognise and remove B cells that help drive the illness.
The process is personalised. T cells are collected from the patient. Scientists add a chimeric antigen receptor in the lab. That receptor helps the T cells latch on to a marker found on B cells. The cells grow in controlled conditions. The hospital then infuses them back into the patient.
How CAR T is tailored
- Blood collection: clinicians harvest a patient’s T cells through a short hospital visit.
- Engineering: a lab adds a receptor so T cells can target B cells associated with MS activity.
- Expansion: the altered cells multiply under strict quality checks.
- Infusion: a single hospital appointment delivers the dose, often within minutes.
- Monitoring: the team watches for side effects and checks immune changes over months.
Scientists are repurposing a cancer breakthrough for autoimmune disease, taking immune cells to places other drugs may miss.
Why this approach has momentum
CAR T has changed care for some blood cancers. Early research has also shown benefit in autoimmune conditions such as lupus, where patients entered remission after a single treatment in small studies. That track record encouraged neurologists and haematologists to collaborate for MS. If the approach produces long periods without relapses, people could reduce or stop other medications.
Experts describe a “one and done” concept. The ideal outcome is a long, quiet phase of disease with no further drug burden. This would matter to parents juggling work, childcare and hospital visits. It could also reduce accumulated disability, which often rises with repeated relapses.
What doctors will watch next
Clinicians will track relapse rates, MRI changes and day‑to‑day function. They will also measure whether B cells stay suppressed without leaving patients unprotected. The trial will gauge durability. A key question is whether benefits last years after a single dose.
The study plans to recruit up to 18 participants worldwide by early 2027, focusing on highly active disease.
Risks and safeguards
No advanced therapy comes without risk. CAR T can trigger cytokine release syndrome, which often looks like a fever and may require hospital care. Some patients report headaches or confusion for short periods after infusion. Blood counts can fall, which increases infection risk. Specialist teams at centres such as UCLH have protocols to manage these effects rapidly.
Selection criteria matter. Trials typically invite people with active relapsing disease who have not gained enough control with robust medicines. Pre‑treatment screening checks infection status, organ function and vaccination history. After infusion, patients receive close follow‑up and may take preventive antibiotics for a period.
What this could mean for people living with MS
About 130,000 people in the UK live with MS. Most are diagnosed between 20 and 40. Many balance unpredictable relapses with work and family. Treatments have expanded in the past decade, yet some still face progression despite highly effective drugs. A single‑visit therapy that resets immunity could reshape planning for education, employment and parenting.
For Ms Henders, success would mean no more emergency dashes while her children watch. It would also mean confidence to teach practical science without worrying about a tremor. Even small gains change daily life. Fewer hospital appointments free up time. Reduced fatigue unlocks family activities at weekends.
Key points at a glance
- Patient: 37‑year‑old biology teacher and mother of two from Hertfordshire.
- Hospital: University College London Hospital delivered the first UK dose for MS in this trial.
- Procedure: a bespoke CAR T-cell infusion lasting around three minutes.
- Goal: long remission with a single treatment, potentially cutting the need for ongoing drugs.
- Trial size and timing: up to 18 participants worldwide, with recruitment running to early 2027.
Context for readers weighing options
CAR T for MS is available only through research pathways at present. People considering trials can speak with their neurologist about eligibility, disease activity, and previous treatments. Standard options remain vital for most patients, including B‑cell therapies, oral medicines and lifestyle measures that support energy, sleep and mobility.
The science moves quickly. Manufacturing times are shortening as centres refine workflow. Researchers are testing different targets on B cells, dosing strategies and eligibility rules. Data from this and parallel studies will inform NHS decisions in the future. Real‑world costs and staffing will influence the pace of adoption if regulators later approve the treatment.
One treatment, years of impact: that is the promise under investigation, not a guarantee, and careful data will decide.
Further reading for understanding the approach
CAR T means chimeric antigen receptor T-cell therapy. “Chimeric” refers to a receptor built from different biological parts. The receptor helps T cells find and remove B cells linked with MS activity. This is different from standard antibodies, which bind to targets directly. Cell therapies can persist in the body and patrol over time, which may explain durable effects seen in other diseases.
A simple way to picture the process is a targeted clean-up team. Engineers train the team to recognise a badge carried by B cells. Once trained, the team returns and focuses on cells bearing that badge. The rest of the immune system continues its normal work, which includes fighting infections and responding to vaccines. Doctors will balance this effect with infection precautions and regular checks.
Practical questions to ask your care team
- How active is my MS on scans and in day‑to‑day symptoms?
- Which existing medicines still make sense for me now?
- Would a research pathway be suitable, and what would follow‑up involve?
- What are the short‑term side effects and how are they managed?
- How might a one‑time therapy change my routine and work commitments?







Three minutes? Wow—if this really quietens relapses, that’s huge for parents like Emily. Fingers crossed 🙂
Only 18 people by 2027 feels tiny. How will they know if benefits truly last years, and what about long‑term B‑cell depletion risks?